Vitamin D Signaling in Liver Cancer: Prevention Strategiesand Future Treatment Prospects - A Literature Review
Tasnia Hossain Lamia, Anid Hassan , Sadia Siraj, SwarnaChakrabarty, Salma Khan
1Department of Cardiology, Square Hospitals Limited, Dhaka, Bangladesh, lforlamia@gmail.com
2Department of Internal Medicine, HMH Jersey Shore University Medical Center, anidhassanmd1993@gmail.com
3Department of Internal Medicine, Texas Tech University Health Sciences Center, Amarillo,sadia_sbmc@yahoo.com
4Department of Internal Medicine, St. Joseph’s Medical Center, Stockon, CA, swarna.nemc@gmail.com
5Bangladesh Medical Association of North America, 20707 Hillside Ave, Jamaica, NY, USA, salmakhan@llu.edu
6Division of Otolaryngology, Loma Linda University School of Medicine, Loma Linda, California, USA
7Department of Internal Medicine, Loma Linda University School of Medicine, Loma Linda, CA, USA
8Center for Health Disparities & Molecular Medicine, Loma Linda University School of Medicine, LomaLinda,California, US
Liver cancer presents a significant global health burden, with most cases diagnosed at advanced stages, leadingtopooroutcomes. Identifying modifiable risk factors is essential for primary prevention. Established risk factors includechronichepatitis B and C infections, heavy alcohol consumption, obesity, diabetes, and aflatoxin exposure. However, manycasesremain unexplained, suggesting dietary and other environmental influences. Vitamin D, traditionally recognizedfor itsroleinbone health, has been increasingly studied for its regulatory effects on inflammation and immune function. Over thepastdecade, research has explored vitamin D’s potential in cancer prevention, particularly in colon, breast, pancreatic, prostate,and liver cancers. This systematic review examines the role of vitamin D in liver cancer, including its metabolism, molecularpathways, treatment possibilities, and limitations.
Keyword: Hepatocellular carcinoma vitamin D 25(OH)D polymorphism liver cancer prevention
Vitamin D Signaling in Liver Cancer: Prevention Strategiesand Future Treatment Prospects - A Literature Review
Tasnia Hossain Lamia, Anid Hassan , Sadia Siraj, SwarnaChakrabarty, Salma Khan
1Department of Cardiology, Square Hospitals Limited, Dhaka, Bangladesh, lforlamia@gmail.com
2Department of Internal Medicine, HMH Jersey Shore University Medical Center, anidhassanmd1993@gmail.com
3Department of Internal Medicine, Texas Tech University Health Sciences Center, Amarillo,sadia_sbmc@yahoo.com
4Department of Internal Medicine, St. Joseph’s Medical Center, Stockon, CA, swarna.nemc@gmail.com
5Bangladesh Medical Association of North America, 20707 Hillside Ave, Jamaica, NY, USA, salmakhan@llu.edu
6Division of Otolaryngology, Loma Linda University School of Medicine, Loma Linda, California, USA
7Department of Internal Medicine, Loma Linda University School of Medicine, Loma Linda, CA, USA
8Center for Health Disparities & Molecular Medicine, Loma Linda University School of Medicine, LomaLinda,California, US
Introduction:
Liver cancer is one of the leading causes of cancer mortality worldwide. As the sixth most common cancer, it ranks the second leading cause. The other cancers for which the data are available are colon, breast, pancreatic, prostate, and liver [1].The common inciting factor for hepatocellular cancer includes Hepatitis B and C viruses. Over-consumption of alcohol is also a cause of this. Other notable elements include aspergillus toxin and metabolic diseases like α1 antitrypsin, hemochromatosis, tyrosinemia, porphyria, and Von Gierke Disease [2].
In its early stages, liver cancer is often asymptomatic, leading to late-stage diagnoses. In the United States, the overall 5-year survival rate for HCC is approximately 20%, a significant improvement from just 3% four decades ago. Survival rates vary depending on disease stage: early-stage diagnosis has a 35% survival rate, while regional spread reduces it to 12%, and distant metastasis lowers it to 3% [3]. Advances in treatment have improved the quality of life even for patients with advanced diseases. Surgery, when feasible, remains the preferred option for better outcomes. Common but often vague symptoms of liver cancer include upper abdominal discomfort, a palpable mass under the right rib cage, right shoulder pain, jaundice, easy bruising or bleeding, nausea, fatigue, and unexplained weight loss [4].
Beyond its role in immune defense, vitamin D influences cellular proliferation, differentiation, autophagy, inflammation, invasion, metastasis, angiogenesis, apoptosis, and miRNA regulation [5]. HCC is particularly prevalent in Japan. Nearly 80 years ago, an inverse relationship between vitamin D levels and cancer incidence was first reported in North America. Subsequent studies in 1980 and 1992 linked low sunlight exposure to increased prostate and colon cancer risks, suggesting that vitamin D may have a protective effect. Further research has reinforced this hypothesis by demonstrating a direct association between vitamin D levels and cancer risk [6]. Given that the liver plays a central role in vitamin D metabolism—serving as the primary site for vitamin D-binding protein and 1,25-dihydroxy vitamin D synthesis—we hypothesized a potential link between vitamin D and liver cancer. Animal models suggest that vitamin D exerts pro-differentiating, antiproliferative, and anti-inflammatory effects on malignant cells, indicating a possible chemopreventive role. Ecological studies have explored the relationship between circulating vitamin D levels and liver cancer risk, though findings remain inconclusive. Therefore, we conducted a systematic review to assess this association further.
Methodology
2.1 Information Source and Keyword Search:
The following items were used in PubMed searches to find relevant articles: “(vitamin D in liver cancer), (vitamin D receptor polymorphism). In addition, the reference lists of reports identified by this search strategy were also searched to select relevant articles.
2.2 Inclusion & exclusion criteria:
Review articles were selected and reviewed. The related literature reviewed the titles and abstracts and was further assessed by examining the full texts. Reports before 2000 were excluded. In animal studies, the pediatric population was excluded from the study.
2.3 Data Collection and Extraction:
An initial search on PubMed for vitamin D in Liver cancer, vitamin D receptor polymorphism, and Vitamin D showed 533 and 289 articles, respectively. Literature research was limited to articles published within the last 24 years. Modifying the article search to the previous twenty-four years resulted in a combined 462 pieces. Adding only review articles, meta-analysis, and other relevant articles that matched our objective showed 40 results. Adding all the keywords and filters, 29 papers were finalized. Six independent authors screened the titles and abstracts of the records to analyze possibly eligible reports. They were standardized using a data extraction table.
Results
A flow chart of included articles is shown in Fig. 1.
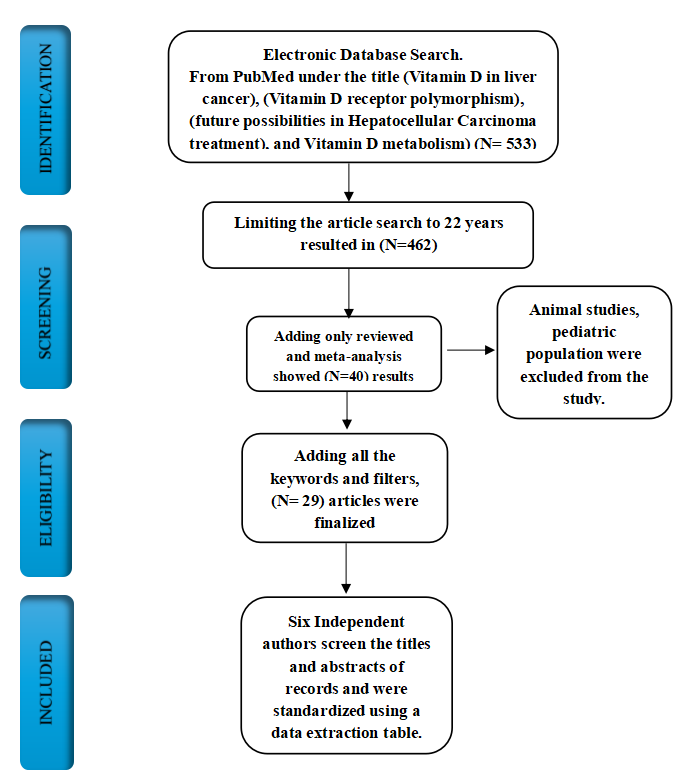
Figure 1. PRISMA flow diagram illustrating the result.
3.1 Vitamin D levels in Liver Cancer:
The global prevalence of vitamin D deficiency is primarily driven by insufficient sun exposure and inadequate consumption of natural dietary sources. If left uncorrected, vitamin D deficiency may contribute to liver cancer progression through β-catenin activation and toll-like receptor 7 signaling pathways. Over the past decade, numerous studies have highlighted a significant association between vitamin D deficiency and liver cancer incidence; however, these findings remain debated. Recent research has suggested several potential mechanisms underlying this relationship, including Notch signaling inactivation, p27 accumulation, and inhibition of tyrosine-protein kinase Met/extracellular signal-regulated kinases [7, 8].
In a meta-analysis, Xiao-Fei Guo et al. proposed that lower circulating vitamin D levels may be linked to liver disease. They demonstrated a significant linear correlation between higher vitamin D levels and reduced liver cancer incidence. However, a key limitation of their study was the predominance of data from Western countries [9].
This meta-analysis further established a strong association between vitamin D levels and hepatocellular carcinoma (HCC), revealing that patients with HCC exhibited remarkably low vitamin D levels. The study suggested that maintaining sufficient serum vitamin D levels could be beneficial for liver cancer prevention. Given the established link between vitamin D deficiency and liver cancer, addressing the widespread deficiency—primarily caused by limited sun exposure and inadequate dietary intake—may be crucial in reducing liver cancer risk [10].
3.2 Vitamin D Binding Protein and Vitamin D Receptor:
Vitamin D binding protein (DBP) is a Group-Specific component (GC). DBP was identified due to its genetic variability across different populations globally [11]. DBP is a multifunctional protein. The primary function of DBP is to carry vitamin D metabolites from the site of synthesis to the site of action [12]. DBP maintains the balance of vitamin D in the blood. DBP's other most crucial function is linked to macrophage activation and neutrophil chemotaxis, which play a role in immune regulation. Furthermore, its circulating levels may be related to the regulation of inflammation. Over time, DBP has been found to have strong associations with various malignancies. Despite this, research is scarce on the role of DBP in hepatocellular carcinoma (HCC), and their relationship remains unclear. Additionally, few studies indicate that DBP expression is crucial in HCC, revealing that DBP can inhibit the malignant progression of HCC [13].
The vitamin D receptor (VDR) plays a significant role in hepatocellular carcinoma (HCC), influencing tumor development and progression through various mechanisms. VDR is expressed in human liver cancer cell lines, particularly HepG2 cells and human HCC specimens. However, VDR expression may be reduced in liver cancer tissue in certain circumstances, providing an escape mechanism from vitamin D effects [13]. The Pathway of the Vitamin D-vitamin D receptor is shown in Figure 2.
.png)
Figure 2: Step-by-step Vitamin D signaling pathway in tumor progression. Note: VD: Vitamin D; VDR: Vitamin D receptor.
3.3 Vitamin D in different subsets of Liver Cancer
3.3.1 Hepatocellular carcinoma and Vitamin D
Hepatocellular carcinoma (HCC) is the predominant primary liver malignancy. The worldwide incidence of HCC has been on the rise, in part due to the increasing prevalence of chronic hepatitis B and C infections. HCC is ranked in the sixth position on a global scale of malignancies, and it is the third most significant cause of cancer-related mortality [14, 15]. Over the past decade, vitamin D has garnered significant attention for its roles in inhibiting cell proliferation, promoting differentiation and apoptosis, reducing inflammation, and regulating immune function. Numerous studies have suggested that vitamin D and its analogs could serve as potential therapeutic targets for various cancers, including HCC. While in vitro and in vivo studies have demonstrated the critical role of vitamin D in carcinogenesis, clinical evidence, and comprehensive reports on its impact on HCC remain limited [16].
3.3.2 Angiosarcoma:
Angiosarcomas are rare neoplasms arising from the endothelial cells lining blood and lymphatic vessels, accounting for only 2–3% of adult soft tissue sarcomas [17,18]. These tumors can develop in various body regions, including the skin, breast, soft tissues, bones, and internal organs, with the skin and breast being the most affected sites.
Hepatic angiosarcoma carries the poorest prognosis among angiosarcomas and has been linked to exposure to vinyl chloride and other chemical carcinogens [16–18]. Its clinical presentation is often nonspecific, with symptoms such as abdominal mass, fatigue, weight loss, and abdominal pain [17,19]. Due to its rarity and aggressive nature, no standardized treatment protocols exist. Chemotherapy provides only palliative benefits, and surgical resection is considered only for cases with a single, localized tumor. However, liver transplantation is not recommended as a treatment option [17].
3.3.3 Cholangiocarcinoma:
Cholangiocarcinoma is a rare malignancy arising from the epithelial lining of the bile ducts, accounting for approximately 2% of all malignant tumors [20]. It can develop at any point along the biliary tree and is classified based on its anatomical location into intrahepatic (within the liver parenchyma), perihilar (around the bile duct hilum), and distal (near the head of the pancreas) subtypes [21, 22]. The primary prognostic factors following surgical resection are margin status and the presence of lymph node metastases [23]. While liver transplantation can provide a cure for specific individuals with perihilar cholangiocarcinoma, it is not an effective treatment for those with intrahepatic or distal cholangiocarcinoma [21,22]. Other observed effects of Vitamin D analogs, include TXNIP expression, Notch signaling inactivation, and p27(kip1)-dependent suppression of pro-inflammatory cytokine secretion [24].
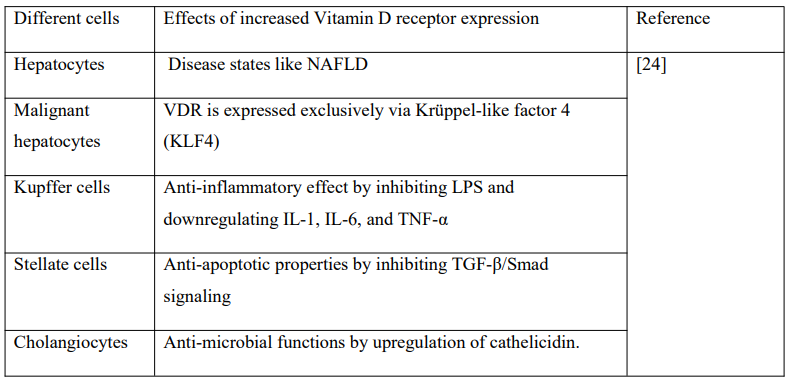
3.4 Vitamin D receptor (VDR) expression in different hepatic cells and malignant hepatocytes:
Normal Hepatocytes have low VDR expression. VDR expression increased in NAFLD and reduced in NASH, which indicates that vitamin D is associated with fatty acid accumulation.
In Kupffer cells, high levels of VDR have an anti-inflammatory effect by inhibiting LPS and downregulating IL-1, IL-6, and TNF-α. In Stellate cells, increased expression of VDR exerts anti-apoptotic properties by inhibiting TGF-β/Smad signaling. In Cholangiocytes, increased expressions of VDR exert anti-microbial functions by upregulation of cathelicidin.
In Malignant Hepatocytes, VDR is expressed exclusively via Krüppel-like factor 4 (KLF4), which has a central role in the activity of vitamin D in cancer management as shown in Table 1 [24].
Table 1: Vitamin D receptor expression in different cells.
3.5 Relationship of vitamin D receptor polymorphism with liver cancer:
A distinct relationship exists between vitamin D receptor (VDR) polymorphisms and liver cancer. The active form of vitamin D, 1,25-dihydroxycholecalciferol, binds to VDR, initiating transcriptional activation that inhibits target genes. These genes are expressed in various tissues, including the liver, colon, breast, ovaries, lungs, bones, kidneys, parathyroid glands, pancreas, T lymphocytes, monocytes, keratinocytes, melanocytes, and cancer cells.
VDR polymorphisms—such as Bsm1, Fok1, Apa1, Taq1, and Cdx2—can be identified using restriction fragment length polymorphism (RFLP) analysis, which stratifies findings based on genetic variation, vitamin D status, and ethnicity. Among these, Bsm1, located at the 3; end of the VDR gene, has been associated with liver cancer. Although Bsm1 is intronic and does not alter the gene sequence during transcription, studies have shown strong linkage disequilibrium with the poly(A) tail in the 3; untranslated region (UTR) in Caucasian and non-Caucasian populations.
Bsm1 has two genotypes: homozygous (BB) and heterozygous (Bb). The BB genotype has been linked to an increased risk of pancreatic cancer but a reduced risk of multiple myeloma. In contrast, the Bb genotype has been associated with an elevated risk of non-Hodgkin & lymphoma, gallbladder cancer, and liver cancer [25].
Future possibilities of vitamin D in HCC treatment:
The continuous development of new techniques and therapeutics offers hope for advancing hepatocellular carcinoma (HCC) treatment. There are unprecedented opportunities to enhance treatment outcomes with various options, including naturally occurring compounds, chemotherapeutics, immunotherapies, and innovative drug delivery methods. Combination therapies promise to improve the efficacy of existing agents [26].
Vitamin D analogs have shown potential in HCC treatment by targeting multiple pathways. Their administration leads to the inhibition of the HGF/c-Met/ERK pathway through c-Met and ERK suppression, upregulation of E-cadherin, and downregulation of Akt. Additionally, they promote p27 accumulation, inducing cell cycle arrest, increase p21 (WAF1/Cip1), reduce HDAC2 activity, and regulate key apoptotic and inflammatory markers such as Bax, p53, caspase 8, DR5, Bcl-2, TLR7, and β-catenin activation. Other observed effects include TXNIP expression, Notch signaling inactivation, and p27(kip1)-dependent suppression of pro-inflammatory cytokine secretion [24].
This article highlights the future potential of vitamin D in HCC treatment. Further research is needed to explore additional strategies for managing the disease, such as vitamin D supplementation and CYP27B1 gene transfection therapy [27, 28]. A recent randomized clinical trial demonstrated that vitamin D supplementation significantly reduced liver cancer mortality, though its impact on cancer incidence was not statistically significant [29].
Limitations:
The study presents findings from animal models but does not stress the importance of conducting human studies to validate the applicability of these results to human liver cancer. The prevalence of vitamin D deficiency differs across geographic regions and is based on sun exposure, complicating the development of universal guidelines. Additionally, the impact of vitamin D receptor polymorphisms on liver cancer risk may differ among various populations and ethnic groups, warranting further research. Although experimental studies suggest a potential role for vitamin D in liver cancer prevention, large-scale clinical trials are necessary to confirm its effectiveness and safety.
In vitro and In Vivo use of Vitamin D and its analogs in Liver Cancer:
A study has demonstrated that in vitro cell division can be modulated by clinical anti-cancer drugs, with the Vitamin D analog Eldecalcitol (ED-71) increasing the percentage of cells in the S phase and reducing those in the G2 phase, like other treatments. Additionally, ED-71 was found to enhance the cell apoptosis rate, confirming its inhibitory effects, although the precise mechanisms remain unclear. In vivo experiments using a mouse tumor model further showed that ED-71 significantly reduced tumor growth and tumor tissue expression, supporting its anti-cancer properties. Moreover, RT-PCR results confirmed that ED-71 inhibited hepatoma cell invasion and migration by increasing E-cadherin and decreasing Akt expression. Further research is needed to investigate additional protein pathways through western blotting and structural analysis techniques [30].
Conclusion:
The study demonstrates a linear association between higher vitamin D levels and a reduced risk of hepatocellular carcinoma. However, future research on a multinational scale involving diverse populations is needed to address heterogeneity and thoroughly evaluate the relationship betweencirculating vitamin D levels and liver cancer risk. While substantial evidence supports vitamin D & anti-inflammatory, immunomodulatory, and anticancer effects, its therapeutic potential is still being explored. Numerous laboratory studies have consistently shown that vitamin D or its Analogs can effectively inhibit the growth of various liver cancer cell lines and reduce tumor size in mice. However, further research is required to understand humans & therapeutic responses better.
Acknowledgement
We acknowledge Research Ambition Group, BMANA for invaluable help planning the design and data selection process. Furthermore, we owe special thanks to Dr. Tahsin Tabassum, Dr. Tonima Ashrafi, Dr. Saiyara Sheikh, and Dr. Utsow Saha for their supervision and contribution to improve the final version of the manuscript.
1. Yang W-S, Zeng X-F, Liu Z-N, Zhao Q-H, Tan Y-T, Gao J, et al. Diet and liver cancer risk: a narrative review of epidemiological evidence. British Journal of Nutrition 2020;124:330–40. https://doi.org/DOI: 10.1017/S0007114520001208.
2. El-Serag HB, Balakrishnan M, Natarajan Y. Epidemiology and Risk Factors of Hepatocellular Carcinoma. Gastrointestinal Oncology - A Critical Multidisciplinary Team Approach 2e, John Wiley & Sons, Ltd; 2024, p. 250–63. https://doi.org/https://doi.org/10.1002/9781119756422.ch 14.
3. Palanca A, Ampudia-Blasco FJ, Real JT. The Controversial Role of Vitamin D in Thyroid Cancer Prevention. Nutrients 2022;14. https://doi.org/10.3390/nu14132593.
4. Wong RJ, Jayasekera C, Jones P, Kanwal F, Singal AG, Ahmed A, et al. An Open-Access, Interactive Decision-Support Tool to Facilitate Guideline-Driven Care for Hepatocellular Carcinoma. Gastroenterology Res 2022;15:297—307. https://doi.org/10.14740/gr1573.
5. Wu D-B, Wang M-L, Chen E-Q, Tang H. New insights into the role of vitamin D in hepatocellular carcinoma. Expert Rev Gastroenterol Hepatol 2018;12:287–94. https://doi.org/10.1080/17474124.2018.1406307.
6. Bertino JR. Landmark Study: The Relation of Solar Radiation to Cancer Mortality in North America. Cancer Res 2016;76:185. https://doi.org/10.1158/0008- 5472.CAN-15-3169. 7. Jeon S-M, Shin E-A. Exploring vitamin D metabolism and function in cancer. Exp Mol Med 2018;50:1–14. https://doi.org/10.1038/s12276-018-0038-9.
8. Yi Z, Wang L, Tu X. Effect of Vitamin D Deficiency on Liver Cancer Risk: A Systematic Review and Meta- Analysis. Asian Pacific Journal of Cancer Prevention 2021;22:991–7. https://doi.org/10.31557/APJCP.2021.22.4.991.
9. Guo X-F, Zhao T, Han J, Li S, Li D. Vitamin D and liver cancer risk: A meta-analysis of prospective studies. Asia Pac J Clin Nutr 2020;29:175–82.
10. Bouillon R, Schuit F, Antonio L, RastinejadF.Vitamin D Binding Protein: A Historic Overview. FrontEndocrinol (Lausanne) 2020;10.https://doi.org/10.3389/fendo.2019.00910.
11. Delrue C, Speeckaert MM. Vitamin DandVitaminD-Binding Protein in Health and Disease. Int J Mol Sci2023;24. https://doi.org/10.3390/ijms24054642.
12. Qin L-N, Zhang H, Li Q-Q, Wu T, ChengS-B, WangK-W, et al. Vitamin D binding protein (VDBP) hijackstwist1 to inhibit vasculogenic mimicry in hepatocellularcarcinoma. Theranostics 2024;14:436—450.https://doi.org/10.7150/thno.90322.
13. Markotic A, Kelava T, Markotic H, Silovski H,Mrzljak A. Vitamin D in liver cancer: novel insightsandfuture perspectives. Croat Med J. 2022Apr30;63(2):187-196. doi: 10.3325/cmj.2022.63.187. PMID:35505652; PMCID: PMC9086812.
14. Waller LP, Deshpande V, PyrsopoulosN.Hepatocellular carcinoma: A comprehensive review.World J Hepatol 2015;7:2648—2663.https://doi.org/10.4254/wjh.v7.i26.2648.
15. Adelani IB, Rotimi OA, Maduagwu EN, Rotimi SO.Vitamin D: Possible Therapeutic Roles in HepatocellularCarcinoma. Front Oncol 2021;11. https://doi.org/10.3389/fonc.2021.642653.
16. Zheng Y, Zhang X, Zhang J, Hui Z, Du W, Li R, etal.Primary hepatic angiosarcoma and potential treatmentoptions. J Gastroenterol Hepatol 2014;29:906–11.https://doi.org/https://doi.org/10.1111/jgh.12506.
17. Chaudhary P, Bhadana U, Singh RAK, AhujaA.Primary hepatic angiosarcoma. EuropeanJournal ofSurgical Oncology (EJSO) 2015;41:1137–43.https://doi.org/https://doi.org/10.1016/j.ejso.2015.04.022.
18. Lahat G, Dhuka AR, Hallevi H, Xiao L, ZouC, SmithKD, et al. Angiosarcoma: Clinical andMolecularInsights. Ann Surg 2010;251.
19. Kielhorn J, Melber C, Wahnschaffe U, AitioA,Mangelsdorf I. Vinyl chloride: still a cause for concern.Environ Health Perspect 2000;108:579–88.https://doi.org/10.1289/ehp.00108579.
20. Selvadurai S, Mann K, Mithra S, BridgewaterJ,Malik H, Khan SA. Cholangiocarcinoma miscodinginhepatobiliary centres. European Journal of SurgicalOncology 2021;47:635–9.https://doi.org/https://doi.org/10.1016/j.ejso.2020.09.039.
21. Razumilava N, Gores GJ. Combinationofgemcitabine and cisplatin for biliary tract cancer:Aplatform to build on. J Hepatol 2011;54:577–8.https://doi.org/10.1016/j.jhep.2010.10.010
22. Khan SA, Emadossadaty S, Ladep NG, Thomas HC, Elliott P, Taylor-Robinson SD, et al. Rising trends in cholangiocarcinoma: Is the ICD classification system misleading us? J Hepatol 2012;56:848–54. https://doi.org/https://doi.org/10.1016/j.jhep.2011.11.015 .
23. Rosen CB, Heimbach JK, Gores GJ. Liver transplantation for cholangiocarcinoma. Transplant International 2010;23:692–7. https://doi.org/https://doi.org/10.1111/j.1432- 2277.2010.01108.x.
24. Markotic A, Kelava T, Markotic H, Silovski H, Mrzljak A. Vitamin D in liver cancer: novel insights and future perspectives. Croat Med J 2022;63:187—196.
25. Gnagnarella P, Raimondi S, Aristarco V, Johansson HA, Bellerba F, Corso F, et al. Vitamin D Receptor Polymorphisms and Cancer. Adv Exp Med Biol 2020;1268:53—114. https://doi.org/10.1007/978-3-030- 46227-7_4.
26. Anwanwan D, Singh SK, Singh S, Saikam V, Singh R. Challenges in liver cancer and possible treatment approaches. Biochimica et Biophysica Acta (BBA) - Reviews on Cancer 2020;1873:188314. https://doi.org/https://doi.org/10.1016/j.bbcan.2019.1883 14.
27. Zhao X, Chen Q, Liu W, Li Y, Tang H, LiuX, etal.Codelivery of doxorubicin and curcuminwithlipidnanoparticles results in improved efficacyofchemotherapy in liver cancer. Int J Nanomedicine2015;10:257–70. https://doi.org/10.2147/IJN.S73322.
28. Heo J, Reid T, Ruo L, Breitbach CJ, RoseS,Bloomston M, et al. Randomized dose-findingclinicaltrial of oncolytic immunotherapeutic vacciniaJX-594inliver cancer. Nat Med 2013;19:329–36.https://doi.org/10.1038/nm.3089.
29. Keum N, Lee DH, Greenwood DC, MansonJE,Giovannucci E. Vitamin D supplementationandtotalcancer incidence and mortality: a meta-analysisofrandomized controlled trials. Annals of Oncology2019;30:733–43. https://doi.org/https://doi.org/10.1093/annonc/mdz059.
30. Ye L, Zhu L, Wang J, Li F. Inhibition of vitaminDanalog eldecalcitol on hepatoma in vitro andinvivo2020;15:663–71. https://doi.org/doi:10.1515/med-2020-0137.